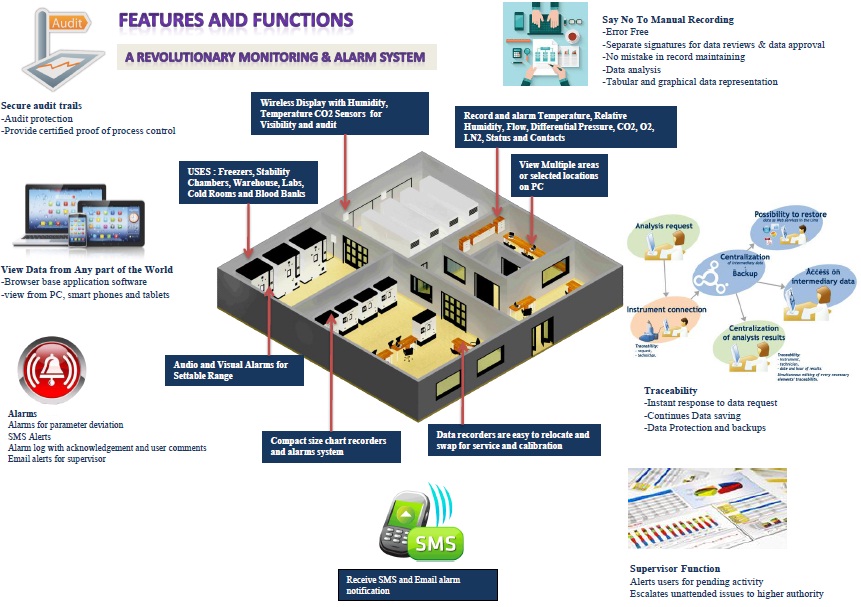
Photostability Chamber
Photostability Chamber manufactured by Meditech Technology for safe testing of specimens
European CE Certified Products Presafe DGM897
Touch Screen and PLC based controller
Inbuilt USB and Data logger
Stability testing for Pharmacy, Cosmetics, Tablets, Beverages and Food
21 CFR Part 11 Ready Software
Web Based Software (SAIFIN53) – Remote Management
25 years of knowledge, expertise and experience
ICH Guideline Q1AR2
At 25°C : RH = 60%
At 30°C : RH = 65%
At 40°C : RH = 75%
Software
Features of 21 CFR part 11 Photostability Chamber
(a) 21 CFR part 11 USFDA compliant software with validation documents.
(b) Multi level security password with aging and history.
(c) Electronic signature.
(d) Secured audit Trail.
(e) GSM alarm system for mobile alert.
(f) Web based Ethernet and WIFI online remote monitoring.
(g)Data of door access security system in software with remote options.
(h)All the events, utility, working status, all the alarms are logged in the software.
(i)Reports of mean Kinetic Temperature Reading.
(j)Records in graphical and in tabular form.
(k)Complete with extensive DQ, IQ, OQ and PQ protocols as per the International guidelines along with calibration certificates.
(l)Works on 230V AC single phase 50Hz (country specific).
Remote
Remote Photostability Chamber and Photostability Room Management – Web Based Software (SAIFIN53)
Access Anywhere Anytime!! Around the Globe
Instantly connect and control your Photostability Chambers and Rooms without installing software. Meditech SAIFIN53 software has gained popularity among operators for its reliability and ease. SAIFIN53 is available remotely via a web browser using internet facility. SAIFIN53 is designed to meet the increasing demands for improved efficiency. This web based software also adds extra capabilities of data logging, user-configuration and features of all alarm notifications via Email and SMS.
Features
(a) Eliminate the need for multiple software Qualifications and installations.
(b) Web based software accessible through browser.
(c)Compatible with server based Operating system, Windows7/8/9/10 and Android .
(d)Ensure one-time software installation on your plants server and access the software anytime, anywhere through a web browser from any Desktop or Laptop (same LAN Network).
(e)View Status, take printouts or execute any of your tasks from anywhere.
(f)Department wise segregation of users, equipment & Data.
(g) Harmonization of reporting format (header,footer,etc) and other such operations through the plant.
(h)Centralized user defined Alarms,alerts and Notifications through SMS & emails.
(i)Configure the type & number of alarms to be sent via email and SMS & configure delay time.
(j)Running time calculation of MKT and Moving Average.
(k)Component running hours to determine the shelf-life of major components.
(l)Real-time display of all connected equipment in single screen.
(m)Automatic data backup facility.
(n)System Diagnosis for troubleshooting via Team Viewer.
(o)Ethernet communication with chambers.
(p)Complying US FDA 21 CFR Part11
Key Features of Photostability Software
(1) A Truly innovative data handling and communication options.
(2)Plug and Play fashion via Ethernet to connect local or plant networked PC or Laptop.
(3)One can view LIVE system parameters and historical graphs as well as receive alarms, reports, data log files over email.
(4)Settings with just a standard browser.
(5)Access live or stored data remotely within the facility (LAN network) of from anywhere in the world.
Test
Together with the help of experts in the pharmacy industry and committees from the pharmaceutical industry and experts from the licensing authorities (such as the FDA) have developed the ICH Guidelines for the harmonization of photostability tests. These guidelines define standardized storage and batch evaluation as well as the time sequence of the required analytic tests.
ICH Q1AR2
Fluorescent tube for day light effect and UV tubes for Ultra Violet ray in accordance to the ICH guidelines requirement of a lightning energy mounting to 1.2 mil lux per hours and integrated UV energy share of 200 Watt Hours per meter square.
As per ICH guidelines :
At 25°C : RH = 60%
At 30°C : RH = 65%
At 40°C : RH = 75%
MEDITECH offers a range of photostability test chambers to meet these regulatory requirements as well as stability testing requirements for other industry segments of Pharmacy, Drugs, tablets, Cosmetic, Food, Beverages, etc.
We offer a centralized solution across all ICH conditions of real-time, intermediate, accelerated & semi-permeable study conditions along with a state-of-the-art 21 CFR Part 11 ready software that provide tools for viewing, trending, alarm management, audit trails, MKT and many other such features.
Construction
(1) Photostability Chamber is manufactured on fully automatic CNC machines thereby giving consistency in performance and aesthetic look.
(2) CFC Free PUF insulation on automatic machine for better accuracy of Temperature and Humidity.
(3) Photostability Chambers are provided with effective air circulation system, refrigeration system, and humidity system.
(4) Full view observation of glass door, chamber illumination and stainless steel perforated trays
(5)Accuracy, Uniformity, Reliability and Economy.
(6)The stability chamber is manufactured as per the cGMP Regulations and designed for low electrical and water consumption.
(7)Stand by refrigeration system and stand by humidity system is provided on request.
Safety
(1) Photostability Chamber is provided with safety cut offs and alarms at centrally located places.
(2) Dedicated Safety system to shut off the humidity system and chamber shut down in case of overshoot or undershoot of temperature or humidity with audio visual alarm.
(3) Thermostatic safety system is provided as an additional safety feature.
(4)Low water level cut off for humidity system.
(5)Door open alarm.
(6) All the alarms and events are logged in the software.
(1) Microprocessor-based 7 inch Touch Screen PID controller. (2) Auto tuning for accurate control of temperature and humidity conditions. (3) Printer interface facility to store data and to print it directly on a printer. (4) Direct % RH electronic capacitor type humidity sensor. (5)Online connection of Touch screen to Computer via Ethernet using TCP IP Protocol for online mapping purpose and data logging. (6)A PLC system for an auto changeover of standby systems and other event logging and monitoring purpose. (7) GSM mobile alert module to give malfunction SMS on mobiles simultaneously (8)Door Access Security System with smart cards and bio-metrics. (9)Network connectivity via Ethernet for web based online remote monitoring. (10) Utility management system provided for continuous machines operations and cross checks. (1) Standard Model(S): Inside S.S.304 & Outside Galvannealed Powder Coated mild steel. (2) GMP Model(G): Inside S.S. 316 with mirror polish & outside with SS 304 matt buff. (3) Temp. Range /Accuracy: 20°C to 60°C /± 0.2°C (4) Temp Uniformity : ± 1°C (5) Humidity Range and Accuracy : 40% to 95 % RH and ± 2% RH (6) Humidity Uniformity : ± 3% RHMonitoring
Control
Features of Photostability Chamber Controller
Humidifier
Meditech patented ultrasonic humidifier MHumid® maintains accurate humidity inside the chamber. Within few seconds the humidity is maintained inside the chamber unlike conventional steam heating system. 95% more efficient than steam heating . Thus saving 95% of electricity
Specification
Model
Litres Inner Dimension
WxDxH cmOuter Dimension
WxDxH cmNo of Trays
MTBBR1 120 45x45x60 65x114x130 2
MTBBR2 200 60x60x60 76x121x130 2
MTBBR3 324 60x60x90 76x121x165 3
MTBBR4 450 60x60x125 76x121x199 4
MTBBR5 600 60x80x125 76x141x199 5
MTBBR6 800 80x80x125 96x141x199 5
MTBBR7 1000 80x80x155 96x141x225 5
MTBBR8 2000 160x75x170 192x126x189
8
MTBBR9 3000 190x80x200 224x131x219
12
Technical
Technical Data Of Proposed Photostability Chamber
About Meditech Photostability Chamber
Meditech has more than 25 years of knowledge , experience and expertise in the field along with feed backs from both customers and distributors. Meditech is highly successful in producing rich features and highly reliable chambers to fulfill customers testing needs.
With Meditech series, one has the ability to apply the laws of nature (temperature and humidity) and allow unknown (possible product defects) to reveal themselves. There shall be increase in reliability and robustness with every test performed on Meditech test Chambers




Recent Comments